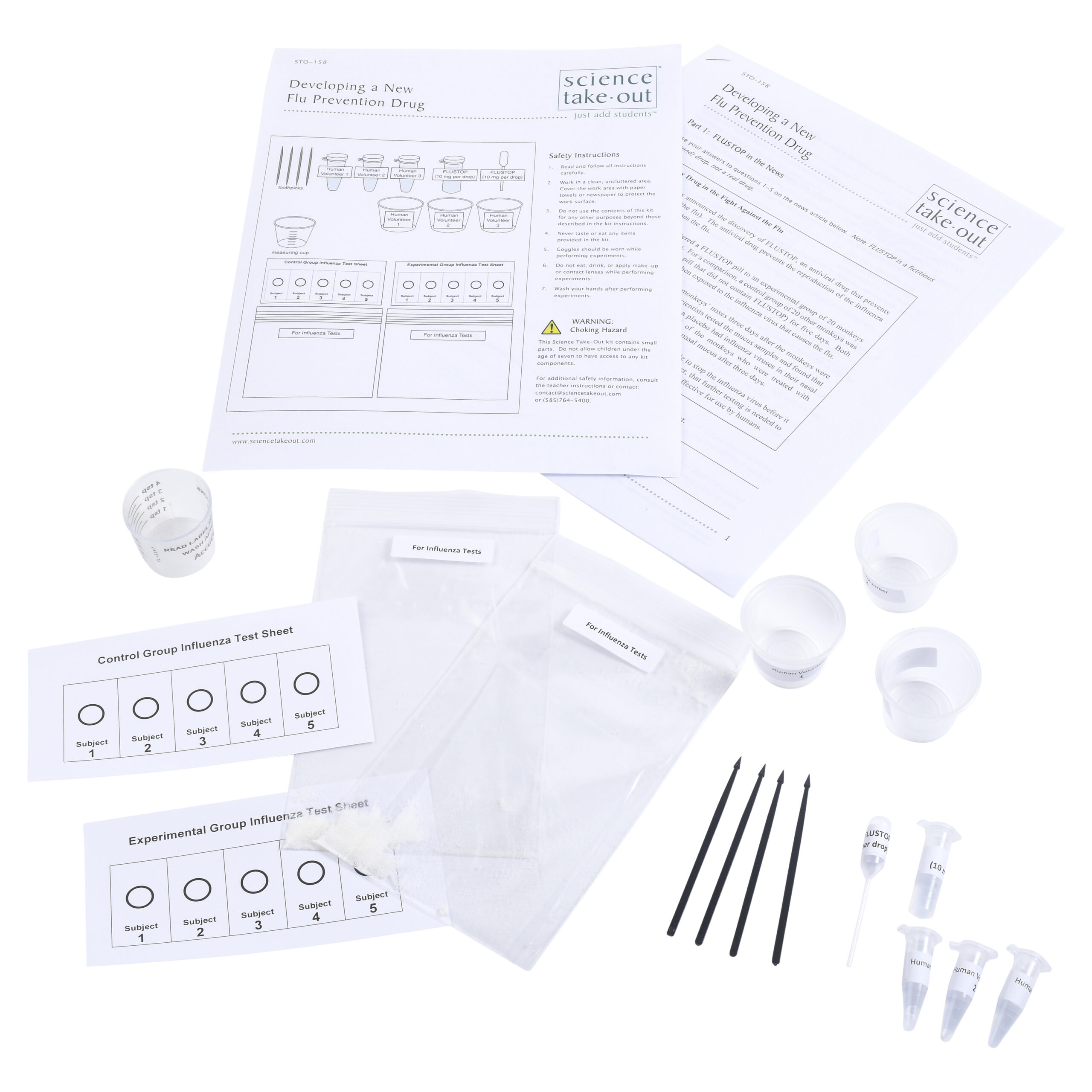
Developing a New Flu Prevention Drug
$18.95 – $137.95
How is a new drug developed and tested to make sure it is safe and effective?
Development of new drugs involves clinical trials – scientific experiments used to determine if a drug is both safe and effective.
- Conduct simulated laboratory tests and analyze data to determine if a new flu prevention drug is safe and effective.
- Learn about the clinical trials process and clinical research.
Kit Includes
- Simulated samples from Human Volunteers 1-3
- Cups for Human Volunteers 1-3
- Toothpicks for stirring
- Tube of “FLUSTOP”
- Plastic dropper for “FLUSTOP”
- Graduated measuring cup
- 2 plastic bags “For Influenza Tests”
- Experimental Group Test Sheet
- Control Group Test Sheet
Quantity Discounts
Kits:
- 1 – 9 kits: $18.95 each
- 10 – 24 kits: $18.00 each
- 25+ kits: $17.06 each
Unassembled:
- 1 – 9 packs: $137.95 each
- 10+ packs: $131.05 each
Refills:
- 1 – 9 packs: $65.95 each
- 10+ packs: $62.65 each
Correlation to Next Generation Science Standards (NGSS) Shop by NGSS »
Performance Expectations:
HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics as well as possible social, cultural, and environmental impacts.
Science & Engineering Practices
Designing Solutions - Evaluate a solution to a complex realworld problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations.
Disciplinary Core Ideas
ETS1.B: Developing Possible Solutions - When evaluating solutions, it is important to take into account a range of constraints, including cost, safety, reliability, and aesthetics, and to consider social, cultural, and environmental impacts.
Crosscutting Concepts
Influence of Science, Engineering, and Technology on Society and the Natural World - New technologies can have deep impacts on society and the environment, including some that were not anticipated. Analysis of costs and benefits is a critical aspect of decisions about technology.